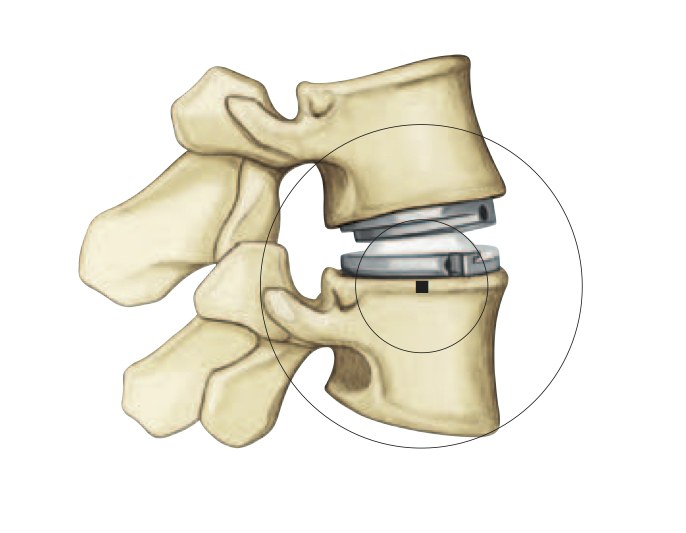
What is ‘Lumbar Disc Replacement’ ?
The ‘intervertebral discs’ are the shock absorbers between the bones of the spine. Unfortunately, they often degenerate – tearing, bursting, or just wearing out. They can cause back or neck pain.
The low back is known as the ‘lumbar’ region.
The usual treatment is rest and painkillers, followed by core muscle (Pilates) exercises. If pain is a problem despite this treatment, one option is to have an operation.
Fusion operation is the conventional approach. In a fusion, some or all of the disc is removed and replaced with bone, which joins to the bones above and below the disc together.
It is the gold standard for comparing all other operations, including the new operation of lumbar disc replacement.


2. Degeneration of the discs adjacent the fusion because it is moving more to compensate [1].
3. Non-union: failure of the bones to join together.
4. Fusion is most reliable when one's own bone is used to join the bones together, but this requires quite a lot of bone. The site from where the bone is taken can be more sore than the spine.


Early research into disc replacement began in the 1960s. Interest was renewed in the 1990s because of the complications sometimes caused by fusion.
Lumbar artificial discs are made of metal and plastic, very similar to successful cervical disc replacements and to hip and knee replacements.
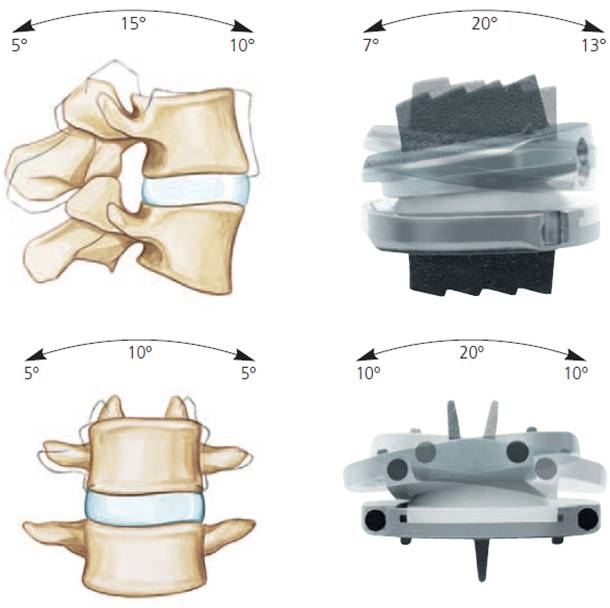
The design is low friction, to reduce energy required to move, and to reduce wear. It provides 20 degrees of forward to backward bending and 10 degrees of side to side bending and 5 degrees of rotation. Unlike spinal fusion, modern designs of lumbar artificial discs can restore the near-normal biomechanics. (Fig. 2)
|
Fig. 2a Centre of rotation of the lumbar artificial disc is located in the normal place, at the superior end plate of the lower segment to keep in line with the facet orientation and movement. |
Fig. 2b Range of motion of the lumbar artificial disc. |


Lumbar disc replacement is a relatively new procedure, so at asia medical specialists we follow the US FDA guidelines:
Chronic, unremitting, discogenic low back pain and disability secondary to single-level degenerative disc disease as medically necessary in a skeletally mature individual when all of the following criteria are met:
- Unremitting low back pain and significant functional impairment is refractory to at least six consecutive months of structured, physician supervised conservative medical management, which includes all of the following components:
- Exercise, including core stabilisation exercises
- Nonsteroidal and/or steroidal medication (unless contraindicated)
- Physical therapy, including passive and active treatment modalities
- Activity/lifestyle modification
- Single-level disc degeneration has been confirmed on CT or MRI.
- The implant will be inserted at an FDA-approved lumbar/sacral level specific to the implant being used.
In other words, the pain is from a single disc and the patient has completed six months of optimal non-operative treatment.
We also respect the FDA guidelines for not performing the operation:
Surgical implantation of a lumbar intervertebral disc prosthesis for any of the following because each is considered experimental, investigational or unproven:
- The planned procedure includes the combined use of a prosthesis and spinal fusion (i.e., hybrid surgery).
- Simultaneous multilevel implantation is planned.
- The implant will be inserted outside of the recommended lumbar/sacral level for the specific implant being used.
- The individual has osteopenia or osteoporosis (T-score < -1.0).
- The individual has a history of prior lumbar fusion.
- There is evidence on imaging studies of any of the following:
- Degenerative spondylolisthesis of Grade 2 or greater
- Infection
- Multilevel degenerative disc disease
- Nerve root compression or spinal stenosis
- Pars interarticularis defect with either spondylolysis or isthmic spondylolisthesis
- Scoliosis
- Severe facet joint arthrosis
- Spinal fracture
- Tumour
- Non FDA–approved lumbar intervertebral disc.
In other words, the pain is from more than one disc or there are other problems in addition to disc pain.


There are two FDA-approved artificial lumbar discs available in Hong Kong: the Charité (FDA-approved 2004) and the Prodisc L (FDA-approved 2006). Both are made by Johnson & Johnson after the companies which developed them were acquired.
I prefer the Prodisc L because it has more stability and good results [4].


The operation is performed under general anaesthetic in hospital by a team of spinal surgeon and vascular surgeon.
A small incision is made in the lower abdomen, and the intestines are moved to the side by the vascular surgeon allowing good access to the front of the spine (Fig. 3). This surgical approach was first performed in 1906 [5] and developed in Hong Kong in the 1950s for the treatment of tuberculosis [6]. With the aid of the operating microscope the spine surgeon can see very clearly, and can completely remove the disc and, if needed, decompress the nerves. A trial implant is placed in the disc space and checked with video X-ray. Selecting the implant which best reproduces the natural spinal curvature produces better results.
After the disc is placed the tissues are sprayed with a layer of anti-adhesive gel to reduce scarring. The surgery usually takes about two hours.
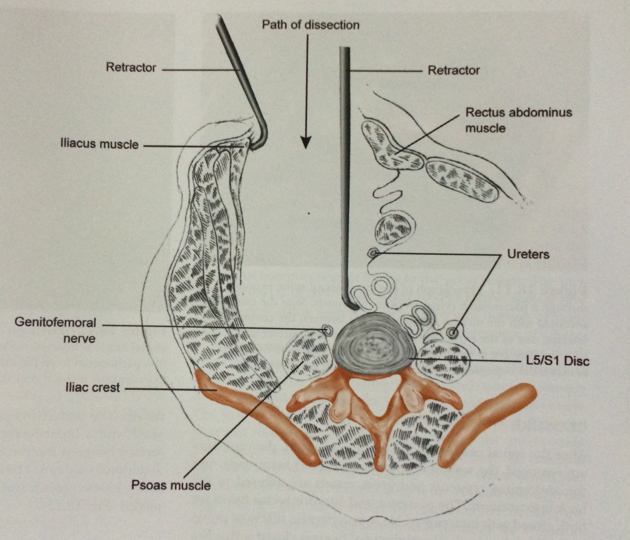
Fig. 3 Anterior approach to the lumbar spine, cross section view.
On the day after the surgery, the patient can walk, and can go home when able to manage stairs independently – usually two days after the operation.


Lumbar disc replacement is relatively new. The latest outcome reports show good results at an average of seven years (range 5 to 11 years) [4]. Many reports are confirming the medium term safety, durability, and effectiveness of lumbar disc replacement. However, so far, compared to fusion, lumbar disc replacement is not much different.
Time will tell if it lives up to our hope that it will provide better long-term results, in particular reducing the problem of adjacent disc degeneration.


- Lumbar disc replacement is a newer technology for treating lumbar disc degeneration causing back pain.
- For selected patients, it may be the best choice of surgical treatment.
- Modern lumbar artificial disc implants provide good medium-term results.
- The risk-benefit ratio for treating back pain is similar to the gold standard lumbar spinal fusion, but it might reduce the chance of future adjacent segment degeneration.


- Zigler JE1, Glenn J, Delamarter RB. Five-year adjacent-level degenerative changes in patients with single-level disease treated using lumbar total disc replacement with ProDisc-L versus circumferential fusion. J Neurosurg Spine. 2012 Dec; 17(6):504-11
- Thavaneswaran P, Vandepeer M. Lumbar artificial intervertebral disc replacement: a systematic review. ANZ J Surg. 2014 Mar;84(3):121-7.
- Rao MJ, Cao SS. Artificial total disc replacement versus fusion for lumbar degenerative disc disease: a meta-analysis of randomized controlled trials. Arch Orthop Trauma Surg. 2014 Feb;134(2):149-58.
- Siepe CJ, Heider F, Wiechert K, Hitzl W, Ishak B, Mayer MH. Mid- to long-term results of total lumbar disc replacement: a prospective analysis with 5- to 10-year follow-up. Spine J. 2014 Jan 18. pii: S1529-9430(13)01475-7.
- Muller,W., Transperitoneale freilegung der wirbelsaule bei tuberkuloser spondylitis. Deutsche Zeitschrift Chirurgie, 1906. 85: p128-135.
- Hodgson, A. and F, Stock, Anterior Spine fusion. Br J Surg, 1956. 44: p 256-257.
All Rights Reserved. Web Design by YSD